- Contact Person : Mr. Wang Quanyong
- Company Name : Beijing Puyishengji Medical Technology Co., Ltd.
- Tel : 86-10-62978132
- Fax : 86-10-62987883
- Address : Beijing,Beijing,Room C101, Jia 2, Tiyuan West Road, Haidian District, Beijing, China
- Country/Region : China
ASD, VSD, PDA CLOSURE/OCCLUDER
Atrial Septal Defect Closure/Occluder, Ventricular Septal Defect (VSD)Occluder , Patent Ductus Arteriosus (PDA) Occluder
Technical principles
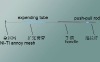
The occluder is made of self-expandable nitinol wire mesh, which is able to comply with the shape of defect parts, so it can be fixed well and do not affect tissues around. Meanwhile, with the flow blocking material sewing in the wire mesh, thrombus can be formed quickly that the endothelial cells could cover on the surface of the occluder to close the shunt completely.
Consisted of metal wire mesh made of nickel-titanium alloy, flow blocking material, stitches, front termination point and back termination point.
Characteristics:
1) The metal mesh of the closure is knitted by nitinol wire(different size with different thickness), which has good memorability and biocompatibility.
2) flow blocking material is good at stoping abnormal bleeding, and it is very thin that decrease the diameter of delivery catheter, which could reduce vascular injury from the operation.
3) Complete product sizes make the occluder more convenient for clinical use.
Applied scope for ASD
1) Age of the patients should be older than or equal to 3 years.
2) Patients are diagnosed as ASD by clinical diagnosis, electrocardiogram, chest film or transthoracic echocardiography, and are considered suitable to deploy closure treatment, with no contraindications.
3) ASD diameter should be greater than or equal to 5 mm, with right ventricular volume overload increases; secundum left-to-right shunt ASD diameter should be less than or equal to 36 mm.
4) The distance from the defect edge extends to coronary sinus, to upper & inferior vena cava or to pulmonary vein should be greater than or equal to 5 mm, but to atrioventricular valve should be greater than 7 mm.
5) Diameter of atrial septum larger than diameter of the closure's left septum part.
6) Not associate with other cardiamorphia must be surgical operated.
Applied scope for VSD
1) Age of the patients should be older than or equal to 3 years.
2) Patients are diagnosed as VSD by clinical diagnosis, electrocardiogram, chest film or transthoracic echocardiography, and are considered suitable to deploy closure treatment, with no contraindications.
3) Meet either criterions listed below:
A: Perimembranous VSD:
a, Simple VSD has hemodynamic effects with heart;
b, VSD with distance between upper edge and aortic right coronary valve ≥1.5mm, without aortic right coronary valve off into VSD or aortic regurgitation;
c, Distance between defect edge and tricuspid valve≥1.5mm, no obvious abnormalities of the tricuspid valve or moderate to severe regurgitation.
B: Ventricular muscular part defect, defect≥5mm
C: Patients with residual shunt after surgery.
Applied scope for PDA
1)Age of the patients should be older than or equal to 6 months, whose weight is heavier than or equal to 4kg.
2)Patients are diagnosed as PDA by clinical diagnosis, electrocardiogram, chest film or transthoracic echocardiography, and are considered suitable to deploy closure treatment, with no contraindications.
3)Meet either criterions listed below:
A. The shunt should be from left to right, not associate with other cardiamorphia must be surgical operated. The narrowest PAD diameter should be bigger than 2.0mm but smaller than 14.0mm.
B Patients with residual shunt after surgery.
4)Whole body in good condition.
5)The patients are willing to do the surgery and sign the information consent form.
ASD, VSD, PDA CLOSURE/OCCLUDER